Throughout its history, NAAF has made research a key focus of its activities. In fact, one of NAAF’s core mission pillars is to support research to find a cure or acceptable treatment for alopecia areata. The success of NAAF’s research endeavors has contributed to the current state of understanding the biological underpinnings of alopecia areata and the hopeful horizon of multiple effective treatments for the disease.
Alopecia Areata Treatment Development Program
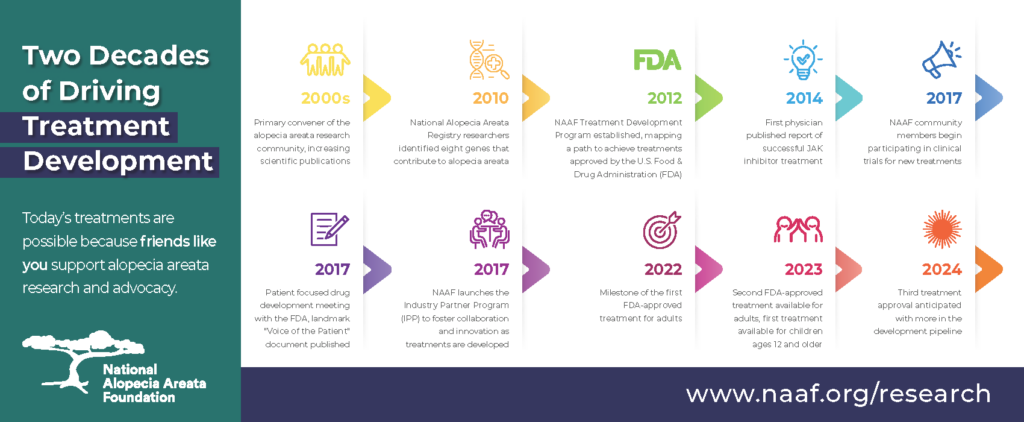
NAAF’s Treatment Development Program (TDP) launched in 2010 with the mission to find a safe and effective treatment or cure for alopecia areata to benefit the 160 million people worldwide who have, have had, or will develop alopecia areata in their lifetime.
Since 1985, based on targeted research aims set forth during biennial research summits, NAAF has awarded 208 research grants totaling more than $5.7 million. Of that, 38 grants totaling $1.65 million have been directed to the line of inquiry that resulted in clinical trials with JAK inhibitors. Those trials are now leading to approved treatments effective in re-growing hair in people with alopecia areata.
 Bringing drugs from discovery to market approval takes an average of 15 years and costs over $1 billion. With NAAF’s early strategic investment of $1.65 million to support the development of a mouse model of the disease, the creation of a registry, and immunogenetic research which led to the investigative trajectory that was so effective , that timeline was shortened considerably. With one approved treatment in 2022 and more on the way, NAAF’s investment in research is paying off for the benefit of the alopecia areata community worldwide.
Bringing drugs from discovery to market approval takes an average of 15 years and costs over $1 billion. With NAAF’s early strategic investment of $1.65 million to support the development of a mouse model of the disease, the creation of a registry, and immunogenetic research which led to the investigative trajectory that was so effective , that timeline was shortened considerably. With one approved treatment in 2022 and more on the way, NAAF’s investment in research is paying off for the benefit of the alopecia areata community worldwide.
Strategically Driven Research Leads to Treatment Breakthroughs
NAAF’s early support of Dr. Angela Christiano’s genetic studies, followed by the formation of the Alopecia Areata Registry, Biobank and Clinical Trials Network (Registry) in partnership with the National Institutes of Health in 2001, proved instrumental in bringing about the groundbreaking 2010 Genome-Wide Association Study (GWAS) published in Nature.
Further genetic research helped unravel underlying immunologic mechanisms that contribute to alopecia areata and identified important immune pathways that could be targeted with drugs approved by the FDA for the treatment of other diseases. In July 2014, Drs. Brittany Craiglow and Brett King published the first case report of complete hair regrowth in a patient with alopecia universalis following treatment with the JAK inhibitor tofacitinib. In September 2014, NAAF advisors and grantees, Drs. Angela Christiano, Raphael Clynes and Julian Mackay-Wiggan, reported in Nature Medicine that the JAK inhibitor ruxolitinib produced near to complete hair regrowth in several patients with alopecia areata. These exciting preliminary findings represented a pivotal moment for alopecia areata research and treatment development.
These triumphs were the result of a long history of Genome Wide Association Studies conducted by Dr. Christiano and supported by NAAF. Drs. Christiano, Clynes, Mackay-Wiggan and other members of the Columbia University team have been active participants in research summits aimed to advance alopecia areata research and the development of safe, effective, affordable treatments. Without quick and easy access to well-characterized samples through the Registry – and the strategic framework of the TDP and associated research summits – these breakthroughs would never have been realized in only a few short years.
TDP continues to drive research by hosting research summits, funding new research through grants, cultivating new researchers with an enduring focus on alopecia areata, and advocating for federal research dollars on Capitol Hill. By convening experts in the fields of hair and skin disease research, clinical care, basic science, immunology, autoimmunity, and industry to distill the science and chart a path forward for the most critical, high-leverage research investments, NAAF is paving the way for a future with multiple safe and effective treatments for alopecia areata.
Treatment Development Timeline Summary
Learn more:
Read the History of the National Alopecia Areata Registry.
Read NAAF’s 2022 Treatment Development Program Impact Report
Watch NAAF’s Treatment Development Program Overview video from 2014